Chromium(VI) — The acidity of chromic acid and the solubility of Chromate salts
Elemental chemistry is the main component of inorganic chemistry, often characterized by its complexity, diversity, and disorderliness. However, beneath the apparent chaos lies a series of interconnected principles in inorganic chemistry, like the framework and concrete that construct a grand and magnificent building. Only through mutual support can stability be achieved.
In elemental chemistry, each substance and property resembles a brick in the memory structure.
Cr, with an electron configuration of 3d54s1, belongs to Group 6B of the fourth period in the periodic table, with its highest oxidation state being +6.
Cr (VI) possesses a high positive charge (+6), a small radius (52pm), and a strong polarizing ability. Aqueous solution readily polarizes water molecules, causing the O-H bonds in water to break, thereby existing in the form of oxygen-containing ions.
Chromic acid
Items | Electric charge | Radius/pm | Acidic medium | oxides | Basic medium |
Ti(IV) | +4 | 68 | TiIO2+ | TiO2 | TiO32– |
V(V) | +5 | 59 | VO2+ VO3+ | V2O5 | VO43- |
Cr(VI) | +6 | 52 | Cr2O72- | CrO3 | CrO42- |
The most common +6 compounds are chromates and dichromates.
H2CrO4 is a moderately strong acid, existing only in dilute solutions, and has not been isolated as a free acid.
As the concentration of H2CrO4 increases, its acidity strengthens. Chromate ions (CrO42-) tetrahedra condense into dimeric species Cr2O72- by sharing oxygen atoms.
Oxyacids of Cr(VI) and Their Ion Transformation in Solution
H₂CrO4 === H+ + HCrO4– Ka1θ=0.18
HCrO4– === H+ + CrO42- Ka2θ=3.2*10-7
As the concentration of Cr(VI) increases and the acidity of the solution strengthens, chromate ions (CrO42-) undergo dimerization to form dichromate dimers (Cr2O72-)
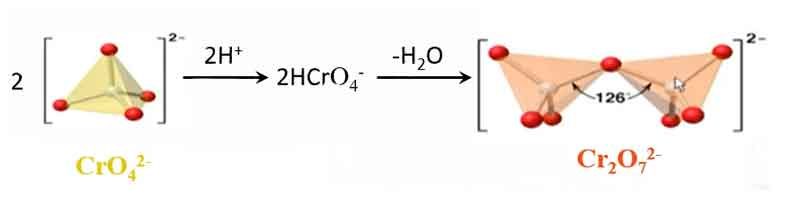
2HCrO4– === H2O+ Cr2O72- Kaθ=1.0*1014
The colorless chromate ion CrO42- forms yellow salts, while the dichromate ion Cr2O72- forms orange-red salts.
In general, chromium (VI) exists in the form of CrO42- in aqueous solutions with a pH greater than 8. In solutions with pH values between 2 and 6, there is an equilibrium between HCrO4– and Cr2O72- , with the equilibrium gradually shifting towards Cr2O72- as the pH decreases. In concentrated sulfuric acid, Cr2O72- is converted to the red compound CrO3.
Therefore, by controlling the solution’s acidity, the equilibrium between CrO42- and Cr2O72- can be manipulated.
It is important to note that Cr2O72- exists as a dimer, and its concentration depends on the solution’s acidity and chromium concentration (VI). The higher the chromium concentration (VI), the greater the concentration of Cr2O72- within the appropriate acidity range. When the concentration of chromium (VI) is less than 10-4 mol∙L-1, no significant amount of Cr2O72- is present within the pH range of 1-8.
H2Cr2O7 is a strong acid and is the dimeric product of H2CrO4. Hence, it is more acidic than H2CrO4.
H2CrO4 === H++HCrO4– H2Cr2O7====H+ + HCr2O7–
Ka1θ=9.55 <
HCrO4– ==== H++ CrO42- HCr2O7– === H+ +Cr2O72-
Ka2θ=3.2*10-7 Ka2θ=0.85
Chromate salts
To date, free H2CrO4 and H2Cr2O7 have not been isolated, but chromate salts and dichromate salts can both exist stably.
The solubility of chromate salts of the same metal ion is less than that of the corresponding dichromate salts. Chromate salts exhibit relatively more sparingly soluble and insoluble salts.
CrO42- : Most are sparingly soluble. Salts with alkali metals (common examples include Sodium chromate and Potassium chromate) and magnesium are easily soluble. Salts with alkaline earth metals(common examples include Calcium chromate and Barium chromate) are sparingly soluble . Salts with heavy metals( Lead chromate)are sparingly soluble.
Cr2O72-: Mostly soluble in water at room temperature Ag2Cr2O7↓
Same metal salt, Dichromate solubility>Chromate solubility
Ag2Cr2O7↓ Ag2CrO4↓
Kspθ=2.0*10-7 2.0*10-12
Adding silver ions (Ag⁺) to a solution dichromate salt (Cr₂O₇²⁻) or chromate salt (CrO₄²⁻) will result in the formation of silver chromate (Ag₂CrO₄↓).
Chromate salts have tetrahedral anions in their solid lattice and aqueous solution.
Similar to Ag+, Ba2+ and Pb2+ also form insoluble chromate salts.
Kspθ
CrO42-+Ba2+====BaCrO4↓ 1.2*10-10
CrO42-+Pb2+====PbCrO4↓ 2.8*10-13
CrO42-+Ag+====Ag2CrO4↓ 2.0*10-12
To shift the equilibrium of CrO₄²⁻ dimerization to the left.
Cr2O72-+2Ba2++H2O →2BaCrO4↓+2H+
Cr2O72-+2Pb2++H2O →2PbCrO4↓+2H+
Cr2O72-+4Ag++H2O →2Ag2CrO4↓+2H+
For the identification of silver ions (Ag⁺), barium ions (Ba²⁺), and lead ions (Pb²⁺)
Therefore, whether adding these ions to a solution containing CrO₄²⁻ or Cr₂O₇²⁻ salts, the resulting precipitates are chromate salts of these ions, not dichromate salts. However, the sparingly soluble chromate salts dissolve in strong acids because as the
acidity increases, CrO₄²⁻ converts to Cr₂O₇²⁻ and dissolves.
Additionally, due to the different properties of various cations, the dissolution and precipitation transformation of chromate salts varies in different solvents.
| Items | NHO3 | HCL | H2SO4 | NaOH |
Soluble | Soluble | BaSO4↓ | ||
Soluble | PbCl2 | PbSO4↓ | Soluble | |
Ag2CrO4 | Soluble | AgCl | Ag2SO4↓ | Ag2O |
2BaCrO4+2H2SO4===2BaSO4 +Cr2O72- +2H++H2O
2PbCrO4+2H+===2Pb2+ +Cr2O72- +H2O
PbCrO4+4OH–===[Pb(OH4)]2- +CrO42-
2PbCrO4+2H2SO4===2PbSO4 +Cr2O72- +2H++H2O
2PbCrO4+4HCL===2PbCl2 +Cr2O72- +2H++H2O
2Ag2CrO4+4HCL===4AgCl +Cr2O72- +2H++H2O
Ag2CrO4+2OH–===Ag2O +CrO42- +H2O
Our company is a factory specializing in producing barium chromate, sodium chromate, potassium chromate, lead chromate, ammonium chromate, and zinc chromate at favorable prices; please get in touch with us for free samples.
